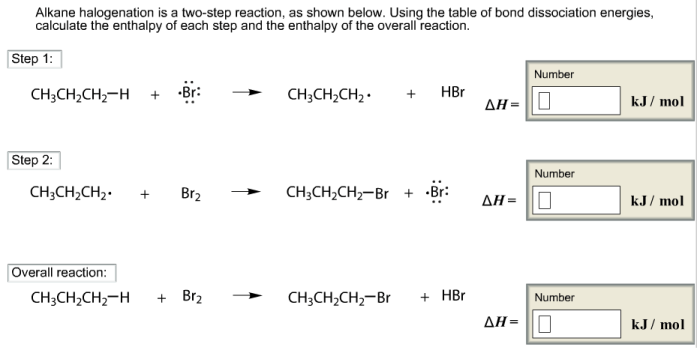
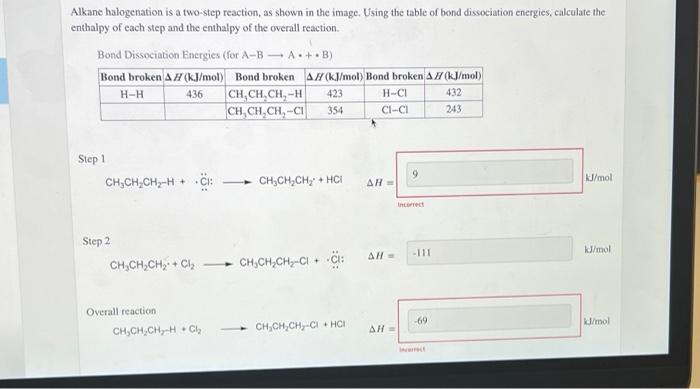
Alkane halogenation is a two step reaction – Alkene halogenation, a two-step reaction, stands as a cornerstone in organic chemistry. This process, involving the addition of a halogen to an alkene, unveils a fascinating interplay of mechanisms and factors, shaping the outcome of countless organic transformations.
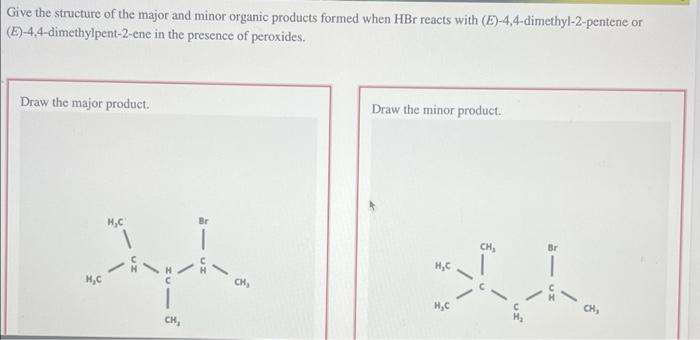
The journey of alkene halogenation begins with the formation of a reactive electrophile, the halogen cation. This electrophile, seeking a nucleophilic partner, encounters the alkene’s pi electrons, initiating a cascade of events that ultimately leads to the formation of a vicinal dihalide.
Along this reaction pathway, free radicals emerge as key players, orchestrating the regio- and stereoselective addition of halogens.
Alkane Halogenation Mechanism: Alkane Halogenation Is A Two Step Reaction
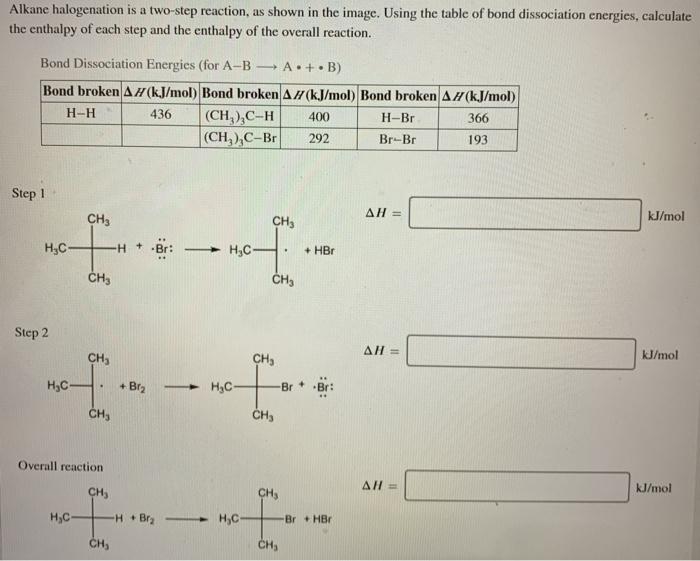
Alkane halogenation is a two-step electrophilic addition reaction involving the addition of a halogen (X2) to an alkane (RH) to form an alkyl halide (RX). The mechanism involves the generation of free radicals, which are highly reactive species containing an unpaired electron.
Step 1: Initiation
The reaction is initiated by the homolytic cleavage of the halogen molecule, resulting in the formation of two free radicals:
“`X2 → 2X•“`
Step 2: Propagation, Alkane halogenation is a two step reaction
The free radical X• reacts with the alkane RH to abstract a hydrogen atom, forming a new free radical R• and HX:
“`R-H + X• → R• + H-X“`
The free radical R• then reacts with another molecule of X2, resulting in the formation of the alkyl halide RX and a new free radical X•:
“`R• + X2 → R-X + X•“`
This step propagates the reaction chain, as the newly formed X• radical can react with another alkane molecule, continuing the cycle.
Key Questions Answered
What is the driving force behind alkene halogenation?
The electrophilicity of the halogen cation, coupled with the nucleophilicity of the alkene’s pi electrons, propels the reaction forward.
How do free radicals influence the course of alkene halogenation?
Free radicals, generated in the reaction pathway, facilitate the regio- and stereoselective addition of halogens by abstracting hydrogen atoms from the alkene substrate.
What factors govern the regioselectivity of alkene halogenation?
The regioselectivity of the reaction is influenced by the stability of the carbocation intermediate formed during the first step of the mechanism.